Background
Olverembatinib, a novel, potent BCR::ABL1 tyrosine kinase inhibitor (TKI), shows strong antitumor activity in pts with CML and Ph + ALL. Here, we report on the safety, efficacy, and pharmacokinetic (PK) profiles of olverembatinib in pts with CML (all phases) and Ph + ALL outside of China, particularly in pts previously treated with ponatinib and/or asciminib.
Methods
Olverembatinib was administered orally once every other day (QOD) in continuous 28-day cycles. In the monotherapy cohort, pts were enrolled after treatment failure on at least 2 prior TKIs and randomized to olverembatinib QOD 30, 40, or 50 mg. In the combination cohort, pts with Ph + B-cell precursor (BCP) ALL or lymphoid CML-BP (CML-LBP) resistant to at least 1 TKI were enrolled and received olverembatinib (30 or 40 mg) QOD in combination with blinatumomab.
Results
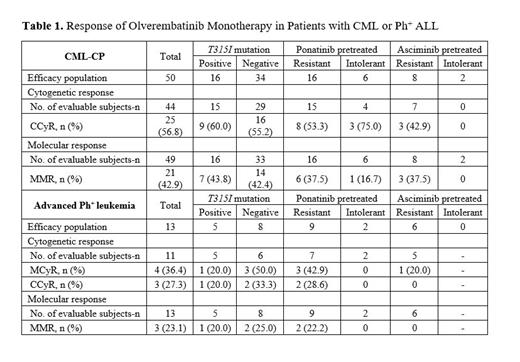
As of June 30, 2023, 76 pts were enrolled, including 57 with CML-CP and 19 with advanced Ph + leukemia. The median (range) age was 54.5 (21-80) years, and 56.6% of pts were male. In all, 11 (14.5%), 23 (30.3%), and 39 (51.3%) pts had received 2, 3, and ≥ 4 prior TKIs, respectively. A total of 52.6% of pts were previously treated with ponatinib, of whom 67.5% were resistant and 25.0% intolerant to the drug, and 7.5% experienced treatment failure for other reasons. A total of 27.6% of pts were previously treated with asciminib, of whom 71.4% were resistant and 19.1% intolerant to the agent, and 9.5% experienced treatment failure for other reasons. At baseline, 32% of pts had T315I mutations, 38% hypertension, and 17.1% other cardiovascular comorbidities. Median (range) treatment duration was 24.1 (0-134) weeks, and PK profiles were similar to historical PK data on Chinese pts. Twelve pts with CML-CP and 7 with advanced Ph + leukemia discontinued treatment: 4 because of AEs, 7 disease progression, and 8 other reasons. A total of 54 of 65 (83.1%) pts who received ≥ 1 dose of olverembatinib experienced any-grade TRAEs. Grade ≥ 3 AEs occurring in ≥ 3 pts (≥ 4.6% incidence) included thrombocytopenia (17%); neutropenia (13.8%); elevated blood creatine phosphokinase (13.8%); leukopenia (7.7%); and anemia and elevated lipase (4.6% each). Ten (15.4%) pts experienced olverembatinib treatment-related serious AEs, of which each were experienced by 1 (1.5%) pt. Two (3.1%) pts discontinued the study because of TRAEs. No TRAE-associated deaths were reported. Olverembatinib showed sustained antileukemic activity in pts with CML and Ph + ALL (Table 1). Among 50 efficacy-evaluable pts with CML-CP, the rate of complete cytogenetic response (CCyR) was 57% (25/44) and major molecular response (MMR) 43% (21/49). Efficacy improved over time; the MMR rate in pts with CML-CP treated for 6 months was 66% and 88% in pts treated for 12 months. Among pts whose disease failed ≥ 4 prior TKIs, CCyR and MMR rates were 57% (13/23) and 42% (11/26), respectively. In pts with CML-CP harboring the T315I mutation, rates of CCyR and MMR were 60% (9/15) and 44% (7/16), respectively, and 55% (16/29) and 42% (14/33) in pts without the T315I mutation. Among evaluable ponatinib-failed pts, 8/15 (53%) achieved CCyR and 6/16 (38%) MMR. Among pts who failed asciminib therapy, 3/7 (43%) achieved CCyR and 3/8 (38%) MMR. Of 8 pts with CML-CP who had prior exposure to both ponatinib and asciminib, 2 (25%) achieved MMR. At 24 months, PFS was 75% (95% CI, 56.1-86.7) and OS was 97.6% (95% CI, 90.8-99.4). Thirteen pts with advanced Ph + leukemia were efficacy-evaluable, of whom 3 (23%) achieved MMR; only 1 of 3 pts with the T315I mutation achieved MMR; the other 2 were also resistant to ponatinib treatment. The median (95% CI) PFS of efficacy-evaluable pts with advanced leukemia was 12.7 (4-19.5) months. In the combination cohort, 2 pts with Ph + BCP ALL received olverembatinib 30 mg QOD with blinatumomab; both achieved CCyR and 1 achieved a negative MRD status after 1 treatment cycle.
Conclusions
Olverembatinib alone or combined with blinatumomab was efficacious and well tolerated in pts with heavily pretreated CML or Ph + ALL . Olverembatinib monotherapy was potent in pts who were either resistant or intolerant to ponatinib and/or asciminib, regardless of T315I mutation status. Olverembatinib may provide an effective new treatment option for pts after failure of 2 or more TKIs. Internal study identifier: HQP1351-CU101. Clinicaltrials.gov identifier: NCT04260022.
Disclosures
Jabbour:Adaptive Biotech: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding. Koller:takeda: Consultancy, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; treadwell therapuetics: Consultancy, Other: safety review committee. Jamy:Ascentage: Other: Advisory Board Participation. Hunter:Sierra Oncology: Membership on an entity's Board of Directors or advisory committees. Mukherjee:Bristol Myers Squibb: Consultancy; Bristol Myers Squibb: Other: Advisory Board; Aplastic Anemia and MDS International Foundation: Honoraria; EUSA: Other: Advisory Board; McGraw Hill Hematology Oncology Board Review: Honoraria; Bristol Myers Squibb: Honoraria; Novartis: Consultancy; Celgene (now BMS): Honoraria; Celgene (now BMS): Consultancy; Celgene/Acceleron: Other: Advisory Board; Novartis: Other: Advisory Board; Blueprint Medicines Corporation: Other: Advisory Board; Genentech and AbbVie: Other: Advisory Board; EUSA: Honoraria; BioPharm: Consultancy; Celgene (now BMS): Research Funding; Novartis: Research Funding; Jazz Pharmaceuticals: Research Funding. Baer:Takeda (Inst): Research Funding; Abbvie (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; FORMA Therapeutics (Inst): Research Funding; Kite, a Gilead company (Inst): Research Funding; Kura Oncology (Inst): Research Funding. Turkina:Fusion Pharma: Speakers Bureau; Pfizer: Other: Travel, accommodation expenses, Speakers Bureau; Novartis: Other: Travel, accommodation expenses, Speakers Bureau. Cortes:Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Research Funding; Gilead: Consultancy; Abbvie: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Research Funding. Guo:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Chen:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Fu:Ascentage Pharma: Current Employment, Current holder of stock options in a privately-held company. Wang:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Jiang:Ascentage Pharma: Current Employment, Current holder of stock options in a privately-held company. Wang:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company. Yang:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Other: Leadership, Patents & Royalties. Zhai:Ascentage Pharma: Current Employment, Current equity holder in publicly-traded company, Other: Leadership (CMO).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal